Chronic Refractory Cough Market is Expected to Boom by 2034 Driven by Advancements in Treatment Options | DelveInsight
The chronic refractory cough market is expected to grow due to a rising diagnosed patient pool, greater awareness and recognition of chronic cough as a distinct condition, limitations of current off-label treatments, and the anticipated launch of emerging therapies with novel mechanisms of action including camlipixant (GSK), haduvio (nalbuphine ER) (Trevi Therapeutics), Taplucainium (Nocion Therapeutics) and others to address unmet needs in refractory patients.
New York, USA, Nov. 24, 2025 (GLOBE NEWSWIRE) -- Chronic Refractory Cough Market is Expected to Boom by 2034 Driven by Advancements in Treatment Options | DelveInsight
The chronic refractory cough market is expected to grow due to a rising diagnosed patient pool, greater awareness and recognition of chronic cough as a distinct condition, limitations of current off-label treatments, and the anticipated launch of emerging therapies with novel mechanisms of action including camlipixant (GSK), haduvio (nalbuphine ER) (Trevi Therapeutics), Taplucainium (Nocion Therapeutics) and others to address unmet needs in refractory patients.
DelveInsight’s Chronic Refractory Cough Market Insights report includes a comprehensive understanding of current treatment practices, emerging chronic refractory cough drugs, market share of individual therapies, and current and forecasted chronic refractory cough market size from 2020 to 2034, segmented into leading markets (the US, EU4, UK, and Japan).
Chronic Refractory Cough Market Summary
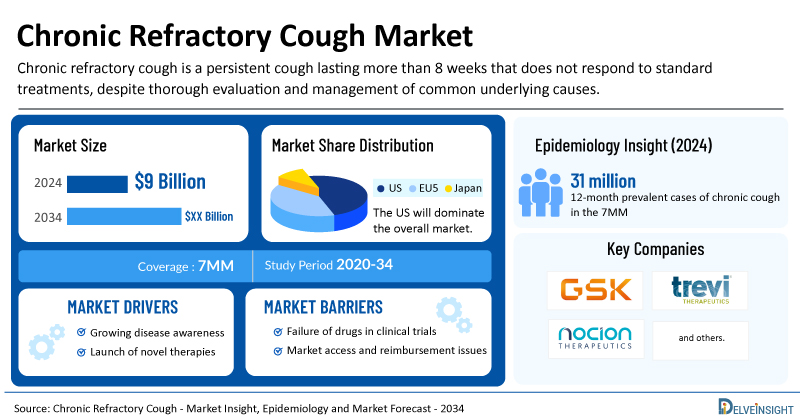
- The market size for chronic refractory cough was found to be USD 9 billion in the leading markets in 2024.
- The United States accounted for the largest chronic refractory cough treatment market size, approximately 50% of the total market size in the 7MM in 2024, compared to other major markets, including the EU4 countries (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- In 2024, the 12-month prevalent cases of chronic cough in the 7MM were estimated at approximately 31 million, of which the US accounted for nearly 13 million.
- Key chronic refractory cough companies, including GSK, Trevi Therapeutics, Nocion Therapeutics, and others, are actively working on innovative chronic refractory cough drugs.
- Some of the key chronic refractory cough therapies in clinical trials include Camlipixant, haduvio (nalbuphine ER), Taplucainium, and others. These novel chronic refractory cough therapies are anticipated to enter the chronic refractory cough market in the forecast period and are expected to change the market.
Discover which chronic refractory cough medications are expected to grab the market share @ Chronic Refractory Cough Market Report
Key Factors Driving the Growth of the Chronic Refractory Cough Market
Rising Chronic Refractory Cough Prevalence
The US accounted for approximately 13 million 12-month prevalent cases of chronic cough in 2024. These numbers are anticipated to rise by 2034, driven by the aging population that has an increased likelihood of developing chronic conditions such as asthma, COPD, and chronic cough.
Advancements in Diagnostics
New diagnostic techniques, such as cough reflex sensitivity testing, high‑resolution computed tomography (HRCT), and improved clinical protocols, improve the precision of diagnosis for refractory cough. Better diagnosis helps in tailored therapy, which in turn supports the adoption of specialized treatments.
Launch of Emerging Chronic Refractory Cough Drugs
The introduction of new therapies, including camlipixant (GSK), haduvio (nalbuphine ER) (Trevi Therapeutics), Taplucainium (Nocion Therapeutics), and others, is anticipated to further bolster the market throughout the forecast period (2025–2034).
Chronic Refractory Cough Market Analysis
Management of refractory chronic cough requires a multidisciplinary strategy combining pharmacological and non-pharmacological approaches. Pharmacologic treatments target neural sensitization and may include neuromodulators, proton pump inhibitors (PPIs) for gastroesophageal reflux, and inhaled corticosteroids (ICS) for airway inflammation. Non-pharmacological interventions focus on symptom control and quality of life, such as speech therapy for cough suppression, cognitive-behavioral therapy for psychological contributors, and airway clearance techniques to reduce mucus and triggers.
Currently, there is no FDA-approved therapy for RCC in the United States. Pharmacologic options mainly address heightened neural sensitivity and include neuromodulators such as opiates (morphine, codeine, tramadol), gabapentin, pregabalin, amitriptyline, and baclofen, along with PPIs, ICS, and antitussives.
LYFNUA (gefapixant) has been approved in Japan (2022) and the European Union (2023) for adults with refractory or unexplained chronic cough. While it is not yet FDA-approved in the U.S., its efficacy has been recognized internationally.
The RCC treatment landscape is advancing, with several promising candidates under development, including GSK’s camlipixant (GSK5464714), Trevi Therapeutics’ haduvio (nalbuphine ER), and Nocion Therapeutics’ taplucainium, among others.
Learn more about the chronic refractory cough treatment options @ Chronic Refractory Cough Treatment Market
Chronic Refractory Cough Competitive Landscape
The launch of emerging therapies, such as camlipixant (GSK), haduvio (nalbuphine ER) (Trevi Therapeutics), Taplucainium (Nocion Therapeutics), and others, is expected to further positively impact the market during the forecast period (2025–2034).
GSK’s Camlipixant is an orally administered, highly selective small-molecule P2X3 receptor antagonist that targets a validated pathway for refractory chronic cough. It is currently being evaluated in two pivotal Phase III trials, CALM-I and CALM-II, with the primary endpoint measuring changes in 24-hour cough frequency at Week 12 (CALM-I) and Week 24 (CALM-II). Topline results are anticipated in the second half of 2025, with GSK planning regulatory submissions and potential approvals in H2 2026.
Trevi Therapeutics’ Haduvio is under development for chronic cough in patients with idiopathic pulmonary fibrosis (IPF) and for renal cell carcinoma (RCC). It has a dual mechanism, acting as a kappa-opioid receptor agonist and a µ-opioid receptor antagonist, allowing it to suppress cough regardless of whether the trigger originates in the lungs or the central nervous system. This dual action blocks cough signals both centrally and peripherally, reducing cough independent of the initial stimulus.
Nocion Therapeutics’ Taplucainium (formerly NTX-1175) is a charged sodium channel blocker designed to silence activated pulmonary nociceptors while minimizing systemic exposure selectively. Unlike P2X3 antagonists, it accesses nociceptors via multiple large-pore channels, providing broad, rapid, and durable antitussive effects, supported by early safety and efficacy data. The Phase IIb ASPIRE trial is assessing once-daily inhaled Taplucainium powder (NOC-110) in adults with refractory or unexplained chronic cough.
The anticipated launch of these emerging chronic refractory cough therapies are poised to transform the chronic refractory cough market landscape in the coming years. As these cutting-edge chronic refractory cough therapies continue to mature and gain regulatory approval, they are expected to reshape the chronic refractory cough market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about new treatment for chronic refractory cough, visit @ Chronic Refractory Cough Medication
What is Chronic Refractory Cough?
Chronic refractory cough is a persistent cough lasting more than 8 weeks that does not respond to standard treatments, despite thorough evaluation and management of common underlying causes such as asthma, gastroesophageal reflux disease (GERD), postnasal drip, or respiratory infections. Unlike ordinary chronic coughs, which often improve once the underlying condition is treated, chronic refractory cough continues to affect patients’ quality of life, leading to physical discomfort, sleep disturbances, social embarrassment, and, at times, psychological distress. Its persistence is often linked to heightened sensitivity of the cough reflex, a condition sometimes referred to as cough hypersensitivity syndrome, which can make even minor stimuli, like cold air or talking, trigger frequent coughing.
Chronic Refractory Cough Epidemiology Segmentation
The chronic refractory cough epidemiology section provides insights into the historical and current chronic refractory cough patient pool and forecasted trends for the leading markets. In the US, the gender-specific cases of chronic cough were nearly 5.1 million and 8 million cases for males and females, respectively, in 2024. Females are more susceptible to chronic cough due to various factors, including anatomical differences in the airways and heightened sensitivity to environmental triggers.
The chronic refractory cough market report proffers epidemiological analysis for the study period 2020–2034 in the leading markets segmented into:
- 12-month Prevalent Cases of Chronic Cough
- Gender-specific Cases of Chronic Cough
- Total Diagnosed Prevalent Cases of chronic refractory cough
- Total Diagnosed Prevalent Cases of Chronic Cough in Idiopathic Pulmonary Fibrosis
Download the report to understand chronic refractory cough management @ Chronic Refractory Cough Treatment Options
| Chronic Refractory Cough Market Report Metrics | Details |
| Study Period | 2020–2034 |
| Chronic Refractory Cough Market Report Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| Chronic Refractory Cough Epidemiology Segmentation | 12-month Prevalent Cases of Chronic Cough, Gender-specific Cases of Chronic Cough, Total Diagnosed Prevalent Cases of chronic refractory cough, and Total Diagnosed Prevalent Cases of Chronic Cough in Idiopathic Pulmonary Fibrosis |
| Chronic Refractory Cough Market Size in 2024 | USD 9 Billion |
| Key Chronic Refractory Cough Companies | GSK, Trevi Therapeutics, Nocion Therapeutics, Merck, Kyorin Pharmaceuticals, and others |
| Key Chronic Refractory Cough Therapies | Camlipixant, haduvio (nalbuphine ER), Taplucainium, LYFNUA, and others |
Scope of the Chronic Refractory Cough Market Report
- Chronic Refractory Cough Therapeutic Assessment: Chronic Refractory Cough current marketed and emerging therapies
- Chronic Refractory Cough Market Dynamics: Conjoint Analysis of Emerging Chronic Refractory Cough Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Chronic Refractory Cough Market Unmet Needs, KOL’s views, Analyst’s views, Chronic Refractory Cough Market Access and Reimbursement
Discover more about chronic refractory cough drugs in development @ Chronic Refractory Cough Clinical Trials
Table of Contents
| 1 | Chronic Refractory Cough Market Key Insights |
| 2 | Chronic Refractory Cough Market Report Introduction |
| 3 | Chronic Refractory Cough Market Overview at a Glance |
| 3.1 | Market Share (%) Distribution of RCC by Therapies in the 7MM in 2024 |
| 3.2 | Market Share (%) Distribution of RCC by Therapies in the 7MM in 2034 |
| 4 | Methodology of Epidemiology and Market |
| 5 | Executive Summary |
| 6 | Key Events |
| 7 | Disease Background and Overview |
| 7.1 | Introduction to RCC |
| 7.2 | Chronic Refractory Cough Signs and Symptoms |
| 7.3 | Chronic Refractory Cough Causes |
| 7.4 | Chronic Refractory Cough Risk factors |
| 7.5 | Mechanism of Chronic Cough |
| 7.6 | Pathophysiology of Chronic Cough |
| 7.7 | Clinical features of RCC |
| 7.8 | Chronic Refractory Cough Diagnosis |
| 7.9 | Chronic Refractory Cough Treatment |
| 7.10 | Chronic Refractory Cough Treatment Guidelines |
| 8 | Epidemiology and Patient Population |
| 8.1 | Key Findings |
| 8.2 | Assumptions and Rationale: The 7MM |
| 8.2.1 | 12-month Prevalent Cases of Chronic Cough |
| 8.2.2 | Gender-specific Cases of Chronic Cough |
| 8.2.3 | Total Diagnosed Prevalent Cases of RCC |
| 8.2.4 | Total Diagnosed Prevalent Population of Chronic Cough in IPF |
| 8.3 | 12-month Prevalent Cases of Chronic Cough in the 7MM |
| 8.4 | Total Diagnosed Prevalent Cases of RCC in the 7MM |
| 8.5 | The US |
| 8.5.1 | 12-month Prevalent Cases of Chronic Cough |
| 8.5.2 | Gender-specific Cases of Chronic Cough |
| 8.5.3 | Total Diagnosed Prevalent Cases of RCC |
| 8.5.4 | Total Diagnosed Prevalent Population of Chronic Cough in IPF |
| 8.6 | EU4 and the UK |
| 8.7 | Japan |
| 9 | Chronic Refractory Cough Patient Journey |
| 10 | Marketed Chronic Refractory Cough Drugs |
| 10.1 | LYFNUA (gefapixant): Merck/Kyorin Pharmaceuticals [not approved in the US] |
| 10.1.1 | Product Description |
| 10.1.2 | Regulatory Milestones |
| 10.1.3 | Other Development Activities |
| 10.1.4 | Clinical Trials Information |
| 10.1.5 | Safety and Efficacy |
| 11 | Emerging Chronic Refractory Cough Drugs |
| 11.1 | Key Cross Competition |
| 11.2 | Camlipixant (GSK5464714): GSK |
| 11.2.1 | Drug Description |
| 11.2.2 | Other Development Activities |
| 11.2.3 | Clinical Trials Information |
| 11.2.4 | Safety and Efficacy |
| 11.2.5 | Analyst Views |
| 11.3 | Haduvio (nalbuphine ER): Trevi Therapeutics |
| 11.4 | Taplucainium: Nocion Therapeutics |
| To be continued in report……. | |
| 12 | Chronic Refractory Cough: Market Analysis |
| 12.1 | Key Findings |
| 12.2 | Key Chronic Refractory Cough Market Forecast Assumptions |
| 12.3 | Chronic Refractory Cough Market Outlook |
| 12.4 | Attribute Analysis |
| 12.5 | Market Size of Chronic Refractory Cough in the 7MM |
| 12.6 | Market Size of Chronic Refractory Cough by Therapies in the 7MM |
| 12.7 | Market Size of Chronic Refractory Cough in the US |
| 12.7.1 | Total Market Size of Chronic Refractory Cough in the US |
| 12.7.2 | Market Size of Chronic Refractory Cough by Therapies in the US |
| 12.8 | Market Size of Chronic Refractory Cough in EU4 and the UK |
| 12.9 | Market Size of Chronic Refractory Cough in Japan |
| 13 | Key Opinion Leader’s Views on chronic refractory cough |
| 14 | Chronic Refractory Cough Market SWOT Analysis |
| 15 | Chronic Refractory Cough Market Unmet Needs |
| 16 | Chronic Refractory Cough Market Access and Reimbursement |
| 16.1 | The United States |
| 16.2 | In EU4 and the UK |
| 16.3 | Japan |
| 17 | Bibliography |
| 18 | Acronyms and Abbreviations |
| 19 | Chronic Refractory Cough Market Report Methodology |
Related Reports
Chronic Refractory Cough Clinical Trial Analysis
Chronic Refractory Cough Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key chronic refractory cough companies, including GSK, Trevi Therapeutics, Nocion Therapeutics, Merck, Kyorin Pharmaceuticals, among others.
Cough in Idiopathic Pulmonary Fibrosis Market
Cough in Idiopathic Pulmonary Fibrosis Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key cough in IPF companies, including NeRRe Therapeutics, Trevi Therapeutics, Algernon Pharmaceuticals, Seyltx Inc., Melius Pharma AB, Cellular Sciences, Emphycorp, among others.
Cough in Idiopathic Pulmonary Fibrosis Clinical Trials Analysis
Cough in Idiopathic Pulmonary Fibrosis Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key cough in IPF companies, including Trevi Therapeutics, Algernon Pharmaceuticals, Melius Pharma, among others.
Asthma Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key asthma companies including GlaxoSmithKline, Areteia Therapeutics, Sanofi, Connect Biopharma, Upstream Bio, Teva Pharmaceuticals, Launch Therapeutics, AstraZeneca, Incyte Corporation, among others.
Severe Asthma Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key severe asthma companies including AstraZeneca plc, GlaxoSmithKline plc, Sanofi S.A., Novartis AG, Roche Holding AG, Teva Pharmaceutical Industries Ltd., Merck & Co., Inc., Boehringer Ingelheim International GmbH, Regeneron Pharmaceuticals, Inc., Johnson & Johnson, Pfizer Inc., Bristol Myers Squibb, Eli Lilly and Company, Vertex Pharmaceuticals Incorporated, Amgen Inc., AbbVie Inc., Genentech, Inc., Gilead Sciences, Inc., Biogen Inc., CSL Limited, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
